Continuing the Conversation
by

What’s Ahead in season two of the podcast series, “The Progress Profile: Alzheimer’s Research in Focus.”
by
What’s Ahead in season two of the podcast series, “The Progress Profile: Alzheimer’s Research in Focus.”
by
For most people, video games are a form of entertainment. For Rhoda Au, Ph.D., professor at Boston University, video games represent something far more profound: a potential window into the human brain.
read moreby
When Dr. Miia Kivipelto launched the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability, better known as the FINGER trial, in 2009, the field of dementia prevention was much different.
“At that time, [the focus was only on] high age and genetic factors,” Kivipelto said. “Now we know that 45% of these risk factors are there throughout the whole life course and that’s why we say that it’s never too early to … prevent dementia.”
Kivipelto, a Finnish neuroscientist and professor who specializes in dementia and Alzheimer’s disease, joined John Harrison, Ph.D., CPsychol, CSci, AFBPsS, an associate professor at Alzheimercentrum, AUmc, Amsterdam, for the Pearson-sponsored podcast series, “The Progress Profile: Alzheimer’s Research in Focus,” to share insights into her research.
read moreby
What decades of research reveal about brain health, early detection, and the future of dementia care
read moreby
What if one of the most overlooked proteins in the brain turned out to hold the key to understanding—and eventually treating — Alzheimer’s disease?
That question was at the center of a recent Pearson-sponsored podcast series, The Progress Profile: Alzheimer’s Research in Focus. Leading Alzheimer’s researcher Dr. Philip Scheltens joined moderator moderator, John Harrison, Ph.D., CPsychol, CSci, AFBPsS, an associate professor at Alzheimercentrum, AUmc, Amsterdam, to explore how tau has moved from a supporting role in the “amyloid hypothesis” to a central player in diagnosis, imaging, treatment, and precision medicine.
read moreby
The growing number of clinical trials and increasing number of drugs in the pipeline are reasons for optimism in the quest for new treatments for Alzheimer’s disease (AD). At the same time, there are concerns that many of the clinical assessments used to evaluate cognition are no longer fit for purpose.
Alzheimer’s researcher Jeffrey Cummings, MD, a neurologist and research professor at the University of Nevada, Las Vegas, notes, “The assessments often date from the 1970s and 1980s, when we had a much less thorough understanding of early disease presentation.” Digital testing offers a solution.
In a new episode of a Pearson-sponsored podcast series, “The Progress Profile: Alzheimer’s Research in Focus,” Cummings joined moderator John Harrison, Ph.D., CPsychol, CSci, AFBPsS, an associate professor at Alzheimercentrum, AUmc, Amsterdam, to share his thoughts on the role of digital testing in Alzheimer’s disease.
read moreby
Tracking cognitive functioning remains a critical part of Alzheimer’s disease (AD) and dementia research. When used properly, COAs offer valuable insights into patients’ conditions, helping researchers track patients’ progress and assess a drug’s efficacy throughout clinical trials.
However, choosing suboptimal scales can be catastrophic for clinical research. The cognitive scales used in the majority of clinical trials for AD, for example, have been pivotal to the failure of 98% of Phase 2 and 3 clinical trials.
In this blog, we’ll discuss how COAs can make or break clinical trial outcomes and share how sponsors can select the optimal scales for their AD clinical research programs.
read moreby
Growth scale values (GSVs) have gained traction as a more precise option for measuring outcomes in clinical trials.
read moreby
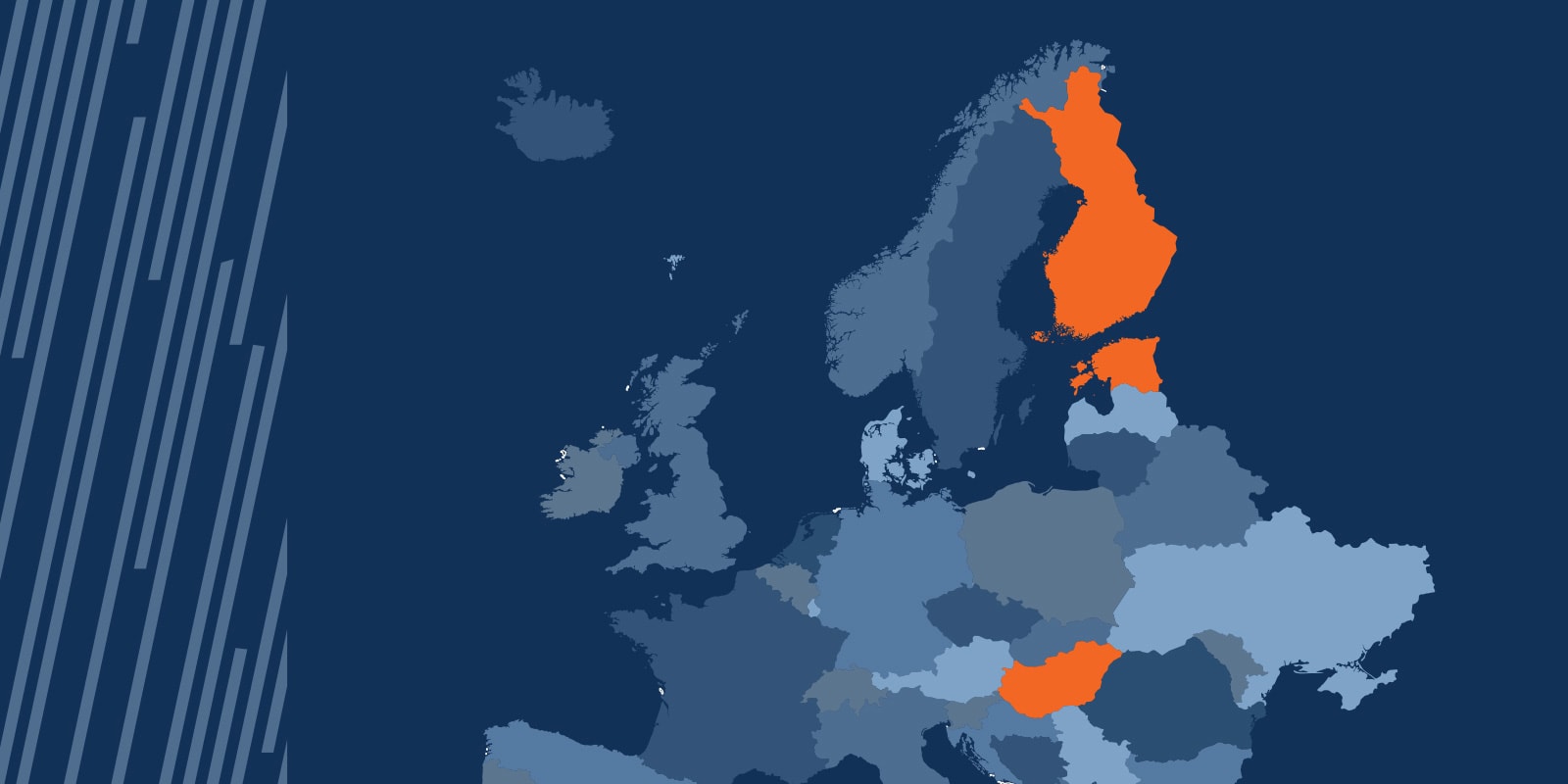
Over the last ten years, the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-3), a leading developmental assessment for children ages 1–42 months, has been referenced as an endpoint in 145 clinical trials (Citeline, 2024). Of those, 6.21% have included sites in the Finno-Ugric countries of Estonia, Finland, and Hungary.
read moreby
Dr. Lynsey Psimas, Ph.D and Dr. Paul Williams PsyD attended CNS Summit 2023 and were fortunate to have the opportunity to present their poster, Precision Matters: An Analysis on How Various Scores Behave When Measuring Change Over Time; Factors That Inform Score Selection for the Best Results”.
read more