Five key insights from leading Alzheimer’s expert, Dr. Philip Scheltens
by

What decades of research reveal about brain health, early detection, and the future of dementia care
When someone finally finds the courage to seek help for memory concerns, they deserve answers, not months of waiting and uncertainty. Dr. Philip Scheltens, a pioneering researcher at the Amsterdam Alzheimer's Centre, has spent decades working to change that reality. In a recent episode of The Progress File: Alzheimer's Research in Focus podcast, Dr. Scheltens shared insights that could transform how we understand, detect, and potentially prevent Alzheimer's disease.
Here are five key takeaways that make complex brain science accessible and hopeful.
1. Your brain’s geography matters: Why amyloid and tau create different symptoms
Think of Alzheimer's disease like a storm system moving through your brain. Dr. Scheltens explains that while amyloid plaques (those sticky protein clumps we often hear about) tend to appear in similar locations for most people, tau protein tangles are much more variable. That's what creates the symptoms we can notice.
"Amyloid pathology occurs in the same place always, but tau pathology is very variable," Dr. Scheltens notes. "The location of tau pathology is much more associated and correlated with whatever cognitive decline and deficit one may measure."
Here's what this means in real terms: You can have amyloid building up in your brain for years (even decades) without noticeable symptoms. But when tau pathology develops in specific brain regions, that's when particular problems emerge:
Tau in the medial temporal lobe → Memory problems
Tau in the parietal lobe → Executive function issues (planning, decision-making)
Tau in frontal/temporal regions → Language difficulties
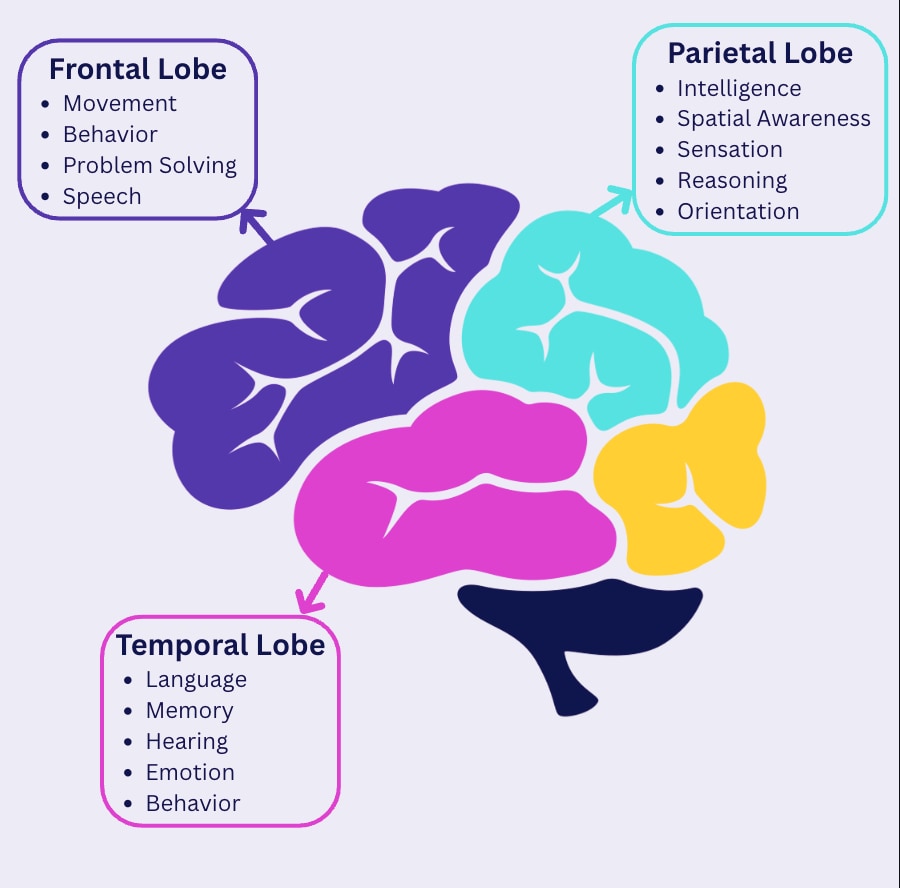
This discovery helps explain why Alzheimer's doesn't look the same for everyone. Some people experience memory loss first, while others might struggle with language or decision-making. Understanding this geography of symptoms could lead to more personalized treatment approaches.
2. The promise and complexity of lifestyle interventions
We've all heard that "what's good for your heart is good for your brain," but Dr. Scheltens offers a nuanced perspective on lifestyle interventions that goes beyond simple platitudes.
His team's research with medical foods—specifically formulated supplements containing omega-3 fatty acids, B vitamins, and uridine—showed promising results. "We showed that actually using this specific supplement had a very interesting effect on cognitive function, not only memory but also executive function, but also notably on hippocampal volume and even whole brain volume," he explains.
The landmark FINGER study further demonstrated that comprehensive lifestyle interventions (combining nutrition, exercise, and cognitive training) can make a measurable difference, particularly for people carrying the APOE4 genetic risk factor.
But Dr. Scheltens is refreshingly honest about the limitations: "The real evidence needs to be found still because for me as a trialist, as a neurologist, I want things to be proven in clinical trials."
His practical advice remains grounded: exercise regularly, follow a Mediterranean diet, don't smoke, maintain a healthy weight. Not because these interventions will definitely prevent Alzheimer's, but because they support overall brain health and give people a sense of agency in their care.
3. Gene therapy: The next frontier for APOE4 carriers
Here's where the conversation gets truly exciting. The APOE4 gene variant significantly increases Alzheimer's risk; having one copy raises your risk about eight-fold, while two copies increase it sixteen-fold. But Dr. Scheltens envisions a future where we don't just manage this risk. We eliminate it.
"I would be much more in favor of having gene therapies actually directing that risk gene specifically and trying to reduce the risk by removing one of these E4 alleles, turning them into an E3 or an E2," he explains.
This isn't science fiction. Companies are actively developing these approaches. The idea is to convert the high-risk APOE4 variant into the more protective APOE2 or neutral APOE3 forms. Such therapy could potentially reduce Alzheimer's risk by 8 to 16 times for carriers.
While these treatments will require extensive testing, the biomarkers we now have for measuring amyloid and tau could help prove their effectiveness in shorter timeframes than traditional clinical trials.
4. The Amsterdam model: Putting patients at the center
What makes the Amsterdam Alzheimer's Centre unique isn't its research alone. It's how seamlessly care and research work together. Dr. Scheltens describes their revolutionary approach: "From the very beginning, we had the idea to have care and research go hand in hand."
Before their model, patients waited months for answers, bouncing between appointments for MRI scans, neuropsychological testing, and blood work. The Amsterdam Centre changed that with their "one-day stop clinic"—comprehensive evaluation and answers in a single visit.
"We could answer that in one day," Dr. Scheltens explains. "That was really very helpful for patients that came from all over the Netherlands."
This approach benefits everyone. Patients get faster answers during an incredibly stressful time. Researchers gather rich, consistent data from 600 new patients annually. Most importantly, new diagnostic tools (from CSF biomarkers to PET scans to blood tests) can be implemented quickly because the infrastructure supports both clinical care and research validation.
5. Blood biomarkers: The game-changer for early detection
Perhaps the most transformative development Dr. Scheltens discusses is the emergence of blood-based biomarkers for Alzheimer's pathology. These tests can detect amyloid and tau proteins in blood samples, potentially identifying disease processes 10-20 years before symptoms appear.
"The biggest change in our thinking, our approach to dementia and disorders that cause dementia, is the fact that we have biomarkers," he emphasizes. "The fact that we have biomarkers for the underlying pathology has helped the field enormously."
This breakthrough enabled the development of new treatments that target the underlying disease process rather than just managing symptoms. While these medications have modest effects, they represent proof of concept that we can intervene in Alzheimer's pathology.
Looking ahead, Dr. Scheltens envisions expanding this biomarker approach to other dementia-causing proteins: "We develop biomarkers for all the different pathologies and develop drugs to target these different pathologies."
The road ahead
Dr. Scheltens' insights reveal a field in rapid transition. We're moving from a one-size-fits-all understanding of Alzheimer's to personalized approaches based on individual pathology patterns, genetic risk, and lifestyle factors. Blood tests are making early detection accessible. Gene therapy offers hope for high-risk individuals. Comprehensive care models are improving patient experiences.
Most importantly, research is accelerating. The biomarkers that took decades to develop are now helping us test new treatments in years rather than decades.
For families facing memory concerns, this research offers both immediate practical guidance and genuine hope for the future. The storm of Alzheimer's disease may be complex, but we're finally developing the tools to track its path—and potentially change its course.
The Progress File: Alzheimer's Research in Focus is available on YouTube. Dr. Scheltens' full conversation with Dr. John Harris offers even deeper insights into the current state and future of Alzheimer's research. You can listen to his episode here.
