Digital Solutions Hub

Digital transformation of Pearson Clinical Assessment
Professionals around the world trust Pearson products to help them make informed decisions in their work to improve lives. Our selection of innovative, research-based digital tools will transform the way you work, improve your workflow, and make you more efficient.
At Pearson Clinical Assessment we understand the pressure that you’re under as a clinical practitioner, and we’re always working to ensure our products are as user-friendly and efficient as possible.
That’s why we’ve gone digital. We’re proud that our digital assessments and tools give you the ability and flexibility to score and administer on screen, plus generate reports on a wide range of assessments with just a few clicks.
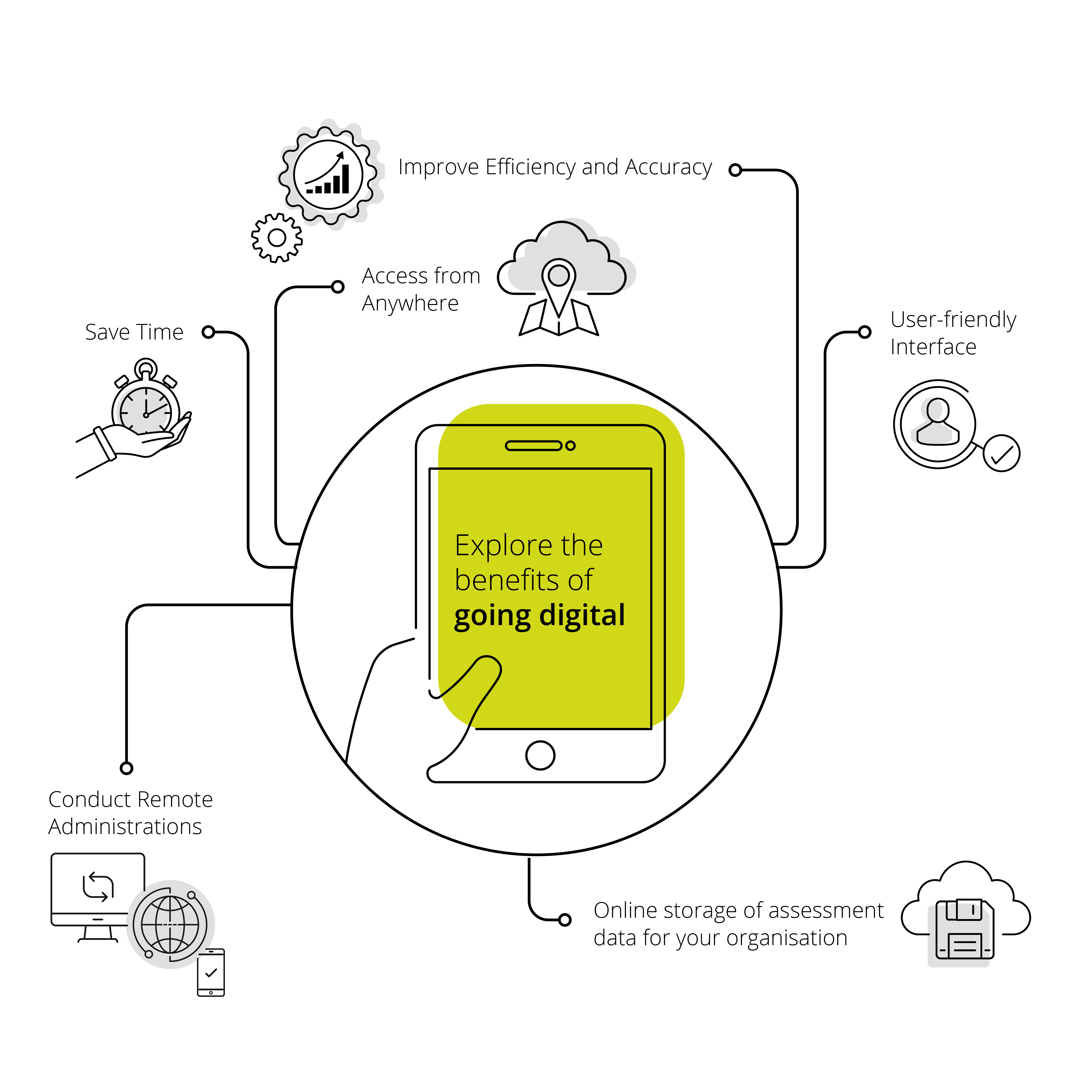
Explore our digital solutions
Your Digital Assessment Journey
What is digital assessment, and how can it benefit you and your practice? Find out below.
 Play
Play
Discover how technology and innovation have revolutionised assessment methods
 Play
Play

European Union’s Medical Devices Directive 93/42EC (MDD) Accreditation
The Q Global and Q Interactive platforms are accredited under the European Union’s Medical Devices Directive 93/42EC (MDD) for the CE Mark as a Tier 1 medical device. They should only be used by licenced and qualified practitioners to assist in making professional decisions and diagnosis. Pearson’s accreditation is valid under MDD until May 2024.
