The role of Clinical Outcome Assessments (COAs) in cognition and dementia research and treatment
How suboptimal Clinical Outcome Assessments (COAs) increase the risk of trial failures and how to curate better assessment strategies to accelerate clinical research.
by

Tracking cognitive functioning remains a critical part of Alzheimer’s disease (AD) and dementia research. When used properly, COAs offer valuable insights into patients’ conditions, helping researchers track patients’ progress and assess a drug’s efficacy throughout clinical trials.
However, choosing suboptimal scales can be catastrophic for clinical research. The cognitive scales used in the majority of clinical trials for AD, for example, have been pivotal to the failure of 98% of Phase 2 and 3 clinical trials.
In this blog, we’ll discuss how COAs can make or break clinical trial outcomes and share how sponsors can select the optimal scales for their AD clinical research programs.
How cognitive endpoint selection can set your trial up for success or failure
While any clinical research around AD and dementia should include scales to assess cognitive dysfunction, selecting suboptimal COAs can hamper clinical research and keep potentially effective drugs from reaching the market.
When designing Alzheimer’s disease (AD) trials, many sponsors incorporate well-established tools such as the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog) or the Clinical Dementia Rating (CDR®) to assess cognitive function. While these instruments have been widely used in clinical research, certain characteristics may limit their utility in detecting meaningful changes in targeted patient populations. For example, some of these tools were not designed for repeated administration, may have limited sensitivity to subtle cognitive changes over time, or focus primarily on episodic memory, providing a narrower view of overall cognitive function.
These constraints can make it difficult to capture the full impact of a treatment, particularly in early or heterogeneous stages of the disease. Expanding the assessment strategy to include tools with broader content coverage and stronger responsiveness can help ensure that trials are positioned to accurately detect treatment-related change.
Newer scales may offer greater insight into cognitive function
As research into cognitive function continues to progress, sponsors have gained access to scales more suitable for clinical research, including:
- Delis-Kaplan Executive Function System (D-KEFS).
- Wechsler Scales (WAIS, WMS, WISC).
- Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).
These scales can assess multiple types of cognition, including various subtypes of memory such as verbal fluency, working memory, visual memory, episodic memory, and immediate as well as delayed memory. D-KEFS, in particular, allows raters to gauge various aspects of executive functioning and attention.
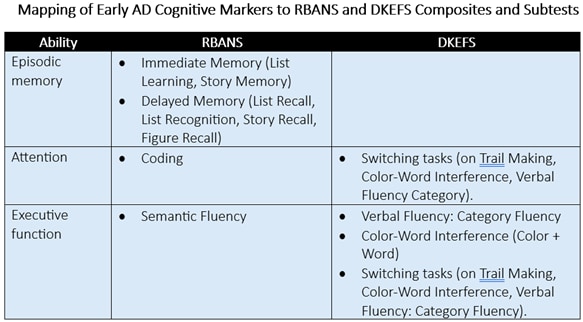
Finally, these scales are also suitable for repeat assessment. That means sponsors gain more insights into participants’ cognitive functioning over time to more accurately measure drug efficacy.
Consider multiple factors when assessing cognition
While COAs play a key role in AD and dementia research, they’re just one aspect to a winning trial strategy.
Sponsors must take a holistic approach to assessing cognition — one that also leverages biomarkers for cognitive decline to validate the results from COAs throughout the trial. Raters can also consider qualitative factors, such as the length of time it takes to complete an assessment or the way the participant organizes their thoughts, which can supplement the results of the assessment to provide broader insights into participants’ cognitive functioning.
Additionally, sites should have discretion on how to assess patients while also centering the patient experience. Lengthy COAs — particularly those administered on-site — can unduly burden patients. Allowing patients to complete assessments digitally, using e-COAs, and selecting the most relevant COA subtests may help sponsors. COA testing, when appropriate, can help sponsors generate the best possible results.
Maximize success in clinical trials with the right COA strategy
COAs can offer valuable insights into cognitive functioning, but they do have limits, and researchers must have a holistic strategy to measure cognitive decline in clinical research and treatment. To succeed, sponsors must leverage the optimal COAs throughout AD and dementia trials, as well as adopt a testing strategy that keeps the patient experience top of mind.